New “biohybrid” machines weave electronics with living cells
Biohybrid tech has long been a staple of science fiction. But researchers now hope to really create these machines, made from both living and artificial parts, that can behave with similar dexterity to living creatures.
With the latest advances in materials science, combined with cutting-edge techniques for genetic engineering, this technology is fast becoming a reality.
Controlling and coordinating the movements of biohybrid devices has remained difficult.
Many designs for biohybrid devices involve sheets of muscle cells that respond to electrical pulses; they can even be genetically engineered to respond to light – a technique named “optogenetics.” This approach has already been used to create biohybrid robots, with coordinated movements including swimming, walking, pumping, and gripping.
Yet so far, controlling and coordinating these movements has remained difficult.
The challenge: To control muscle cells using electricity, researchers use metal electrodes, which must be invasively implanted through delicate living tissues; the electrodes also can’t discriminate between the cells they activate with their pulses.
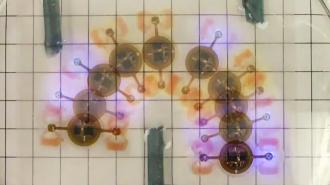
The new “biobot” combines living tissue with a soft, artificial skeleton.
While optogenetic cells — specially modified to respond to light — can help to overcome these issues, the light sources used to control them need to be placed very close to the muscles being targeted.
This takes up valuable space and makes it far more difficult to execute complex movements, which involve highly specific groups of muscle cells. In either case, these devices have to be powered using bulky batteries and connecting wires, making them impractical outside of the lab.
Integrating optoelectronics: According to one team of US researchers, a solution to these challenges could lie with optoelectronics: electronic devices specially designed to find, detect, and control light.
Led by Rashid Bashir at the University of Illinois at Urbana-Champaign, the team demonstrated this idea with a new “biobot,” which combines living tissue with a soft, artificial skeleton.
The researchers could even use gaming controllers to manually guide the biobot through an obstacle course.
This skeleton was made from a 3D-printed hydrogel — a porous polymer that closely mimics the properties of biological tissues. Bashir and his team then seeded this skeleton with optogenetic muscle cells, first grown in mice. These cells were carefully cultivated to grow into a fully biological actuator.
Using laser cutting, the researchers then manufactured an intricate optoelectronic circuit out of a stack of copper sheets. This circuit could pick up radio commands using a receiver coil, then carefully regulate the energy they contained to power a set of microscopic LEDs – making the whole setup completely wireless and battery-free.
Finally, the circuit was coated in a waterproof layer to separate its inorganic components from the rest of the biobot, and then simply slotted into the hydrogel skeleton.
Manoeuvring and assembling: To demonstrate their biohybrid technology, Bashir’s team wrote software to translate user instructions into specific patterns in the light produced by its micro-LED panels.
This caused specific portions of the muscle-cell structure to contract, in ways corresponding to movements, including walking, turning, and “ploughing.”
With their setup, the researchers could even use gaming controllers to manually guide the biobot through an obstacle course, and assemble into formations.
New possibilities for biohybrids: Bashir and his colleagues now hope that future improvements to their design could enable biohybrid machines to combine movement with sensing and on-board computing.
Owing to the power of their optoelectronic circuits, these devices could respond to a wide array of stimuli in their surroundings: from light and heat to the presence of complex chemicals and biomolecules.
It could help doctors to perform minimally-invasive surgeries.
They could also enable researchers to push and transport tiny objects they encounter with near-unprecedented accuracy. Alternatively, biohybrids with on-board computers could be programmed to carry out complex tasks, and make important decisions for themselves.
This technology would be especially groundbreaking in medicine, where it could help doctors to perform minimally-invasive surgeries, and even detect the characteristic chemical signatures of cancer.
Altogether, Bashir and his colleagues hope their results could represent promising early steps towards a new generation of biohybrid machines. For applications across engineering, biology, and medicine, the possibilities presented by weaving advanced electronics with living tissues may one day prove to be practically limitless.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].