FDA approves first-of-its-kind blood cancer treatment
The FDA has approved Johnson & Johnson’s Tecvayli, a new blood cancer treatment designed to help people with hard-to-treat multiple myeloma.
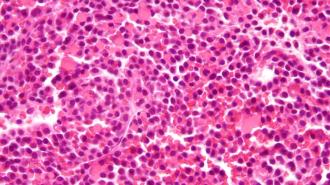
The challenge: Multiple myeloma is a deadly type of blood cancer that affects plasma cells in bone marrow. This can lead to problems fighting infections, weakened bones, kidney dysfunction, and other secondary complications.
Even if an initial treatment is effective, multiple myeloma usually comes back.
Every year in the US, about 34,500 people are diagnosed with multiple myeloma, and the standard treatment options are surgery, radiation therapy, and chemotherapy.
While these can send the cancer into remission, it usually comes back — at that point, a patient is said to have “relapsed multiple myeloma.” If a patient’s cancer doesn’t respond to treatment at all or stops responding to it, it becomes “refractory multiple myeloma.”
Overall, the average five-year survival rate in the US has been about 55% in recent years.
What’s new? J&J’s blood cancer treatment is now approved to treat people with relapsed or refractory multiple myeloma who’ve already received at least four other lines of therapy.
These four lines must include three specific types of treatments: a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
The J&J drug, Tecvayli, is a different kind of treatment, called a bispecific antibody.
J&J’s blood cancer treatment brings cancer cells and T cells together to help the immune system recognize and destroy tumors.
These antibodies can bind to two different targets at once — in the case of Tecvayli, those targets are BCMA (a protein found in high numbers on multiple myeloma cancer cells) and CD3 (a protein found on the immune system’s T cells).
By bringing cancer cells and T cells together, the blood cancer treatment helps the immune system recognize and destroy tumors, Saad Usmani, a senior investigator on an open-label, single-arm phase 2 trial, told the National Cancer Institute.
The trial involved 110 people — all of whom had previously received at least three lines of treatment for their multiple myeloma — and 61.8% of them saw their tumors shrink in response to Tecvayli.
Looking ahead: Now that the FDA has approved Tecvayli, J&J can begin making it available to people with hard-to-treat multiple myeloma — a company spokesperson told Reuters they expect the med to be ready for shipment around November 4.
The spokesperson also said the drug is expected to have a list price of $39,500 per month. Patients will need a 9- to 10-month course, which means the total cost will likely be between $355,500 and $395,000 (though insurance will likely cover most of the bill).
In addition to the high cost, the new blood cancer treatment also carries the risk of serious side effects, including cytokine release syndrome — that’s likely why it’s only approved for patients who have tried several other treatments first.
The cost and risk may well be worth it to patients who’ve run out of effective treatment options, though.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].