Neurosoft CEO says new brain implant is “basically 1,000 times softer” than anything on the market
A new kind of flexible brain implant, developed by Neurosoft Bioelectronics, has been tested in humans for the first time. While more research is needed to prove its safety and efficacy, the device could give us a better way to analyze the brain — or even stimulate it with precise pulses of electricity.
The challenge: Almost everything you think and do is encoded in tiny electrical signals in your brain, so recording this activity can provide valuable insights into your health. In some cases, doctors can even treat patients by stimulating parts of their brain with electricity.
“You want to avoid interfacing [the brain] with something that is very rigid.”
Nicolas Vachicouras
While some recording and stimulation can be done non-invasively, using magnetic fields or EEG caps, brain implants are the most accurate and precise tools available. Rigid electrode arrays can damage the brain, though, leading to scar tissue that degrades the implants’ function over time.
“You [can] think about the brain as panna cotta or tofu — it’s a very, very soft tissue,” Nicolas Vachicouras, CEO and founder of the medtech startup Neurosoft, told Freethink. “You want to avoid interfacing it with something that is very rigid, that can compress it or that could damage blood vessels.”
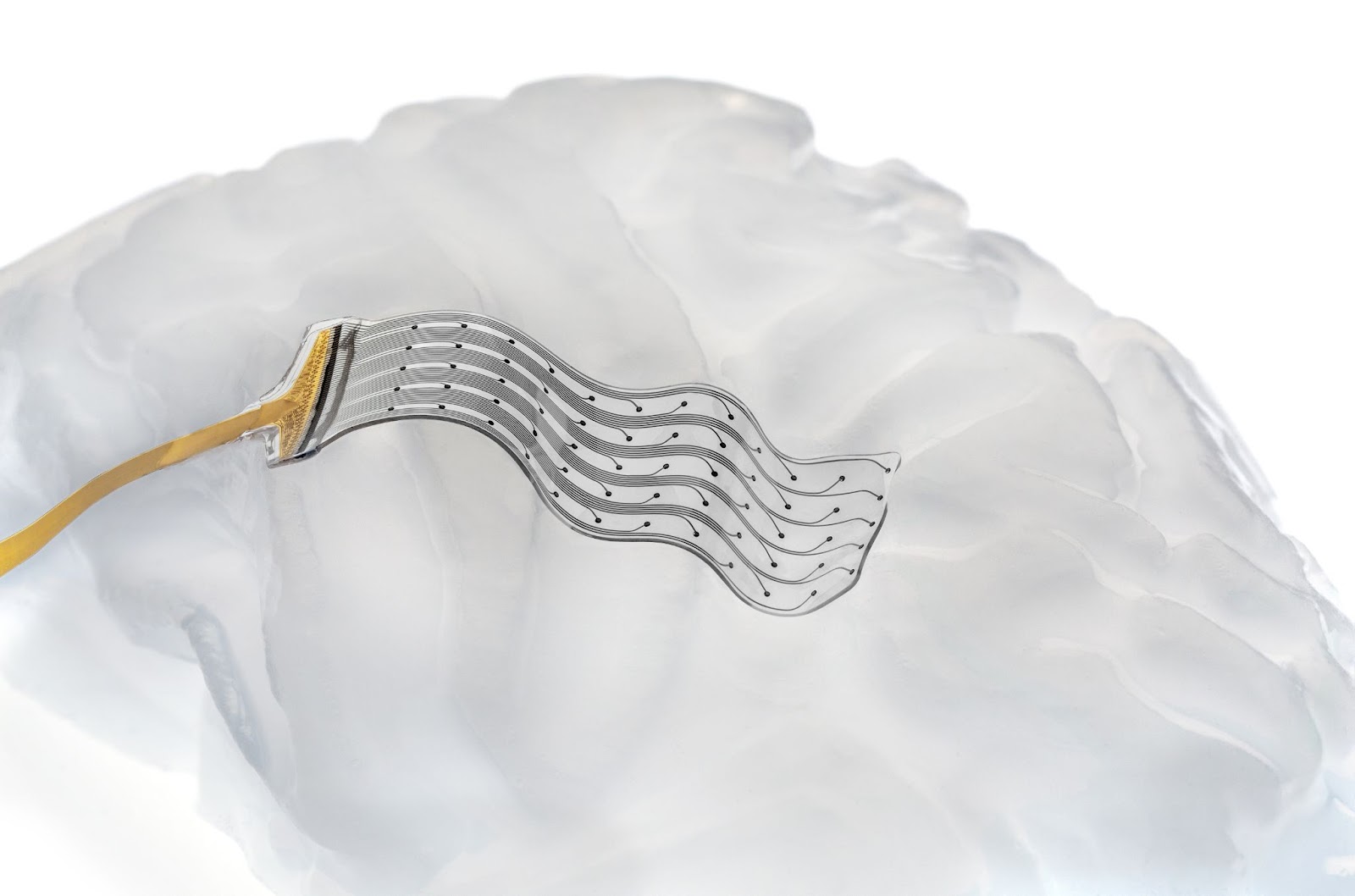
A softer approach: Neurosoft is developing a new type of soft, flexible brain implant that sits on the surface of the brain, just below the skull. Once in place, the implant’s electrodes can be used to record brain activity or deliver stimulation.
“Two things are unique about our electrode,” said Vachicouras. “One is that the materials we use are a lot softer than what’s currently available on the market — basically 1,000 times softer. They’re very elastic and about two times thinner, too.”
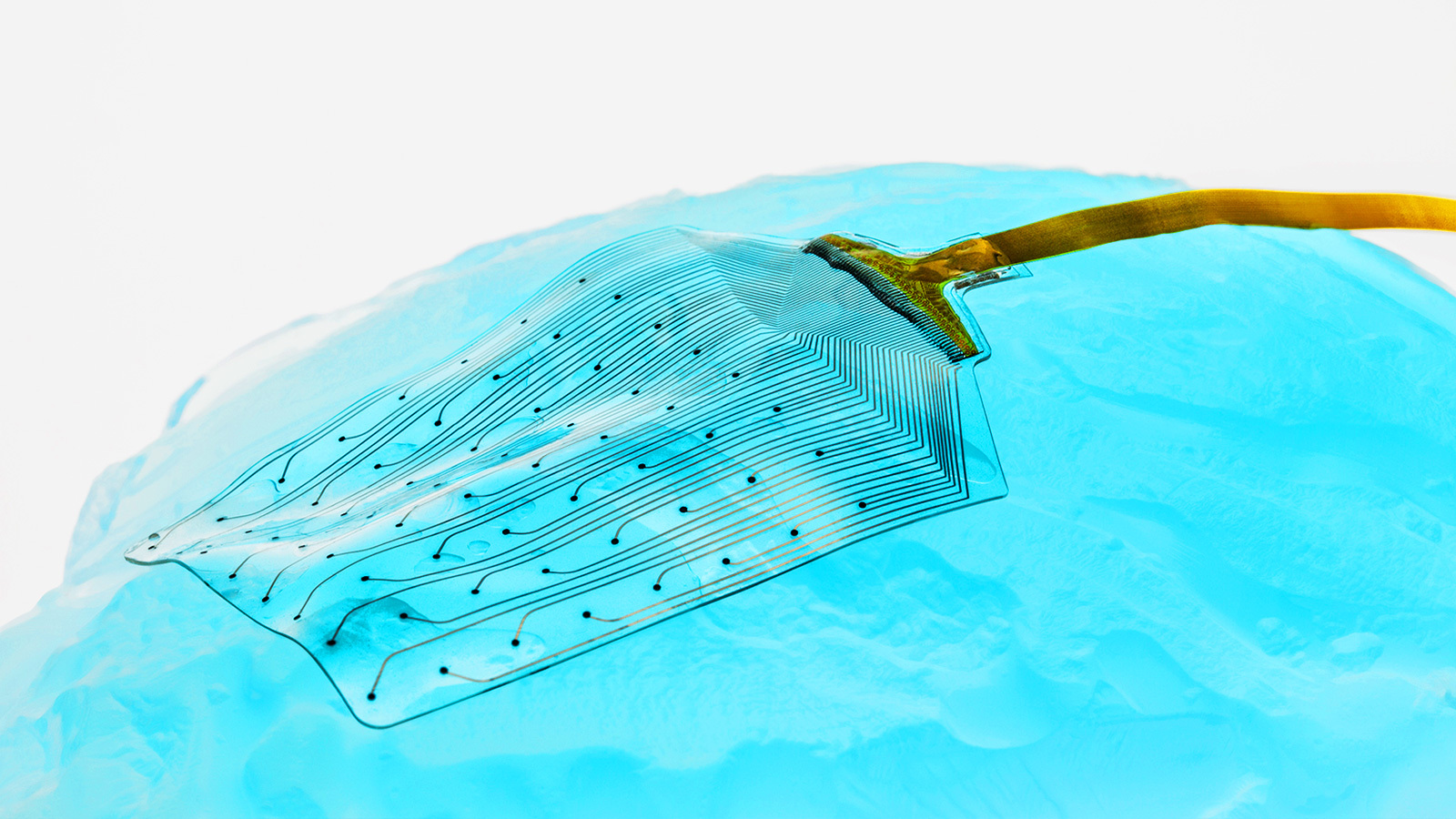
Because the implant is so soft, thin, and flexible, it can conform to the brain’s ridges and folds more closely than a standard electrode array, giving researchers access to parts of the brain that might otherwise be unreachable.
“The other aspect is the size of the sensors — they are much smaller than what’s currently on the market,” said Vachicouras. “The typical analogy I use here is a TV screen with more pixels. If you have more pixels, you have a clearer image of what you’re seeing on the screen.”
“If you have more sensors closely together, you can have a better picture of what you’re recording from the brain,” he continued. “You’re also much more precise when you stimulate because you only stimulate where the sensor is instead of stimulating a big brain region.”
“It was very critical for us to show that it works in a real environment.”
Nicolas Vachicouras
Out of the lab: In August, Neurosoft announced that it had tested its flexible brain implant in humans for the first time, using it to record brain activity while three people were having open brain surgery (one to have a brain tumor removed, and the other two to treat epilepsy).
In this first-in-human trial, the implants were only placed on the brain for the duration of the surgeries, but in animal studies, they’ve proven safe and effective for up to nine months. Testing them in people, even for a short period, was an important milestone, though.
“It was very critical for us to show that it works in a real environment,” said Vachicouras. “In the operating room, you have a lot of electronic equipment that can generate noise that could potentially hide the information you’re trying to record from the brain.”
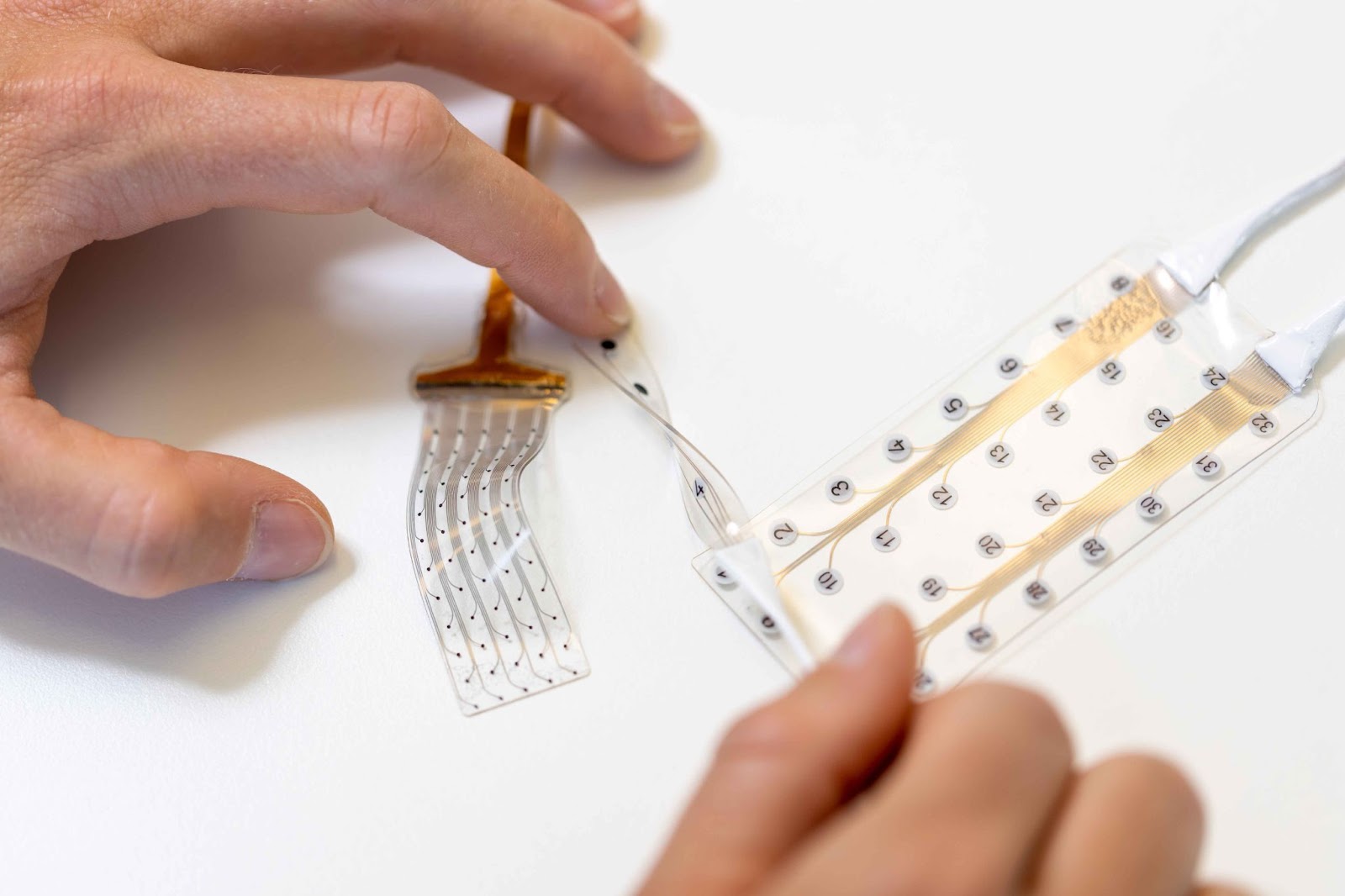
Since then, Neurosoft has trialed the device in one other patient, Vachicouras told Freethink, and the company is now using insights from those first tests to update the implant — which it manufactures in-house — before trialing it in another six patients.
“It’s little things, like the length of the cables and where we should have the connector, that just make it easier to handle,” he explained. “We’re also going to increase the number of electrodes from 30 to 64 and slightly increase the density, so how close they are from each other.”
Looking ahead: In addition to making their flexible brain implant available to other research groups, including one developing a brain-computer interface, Neurosoft has two applications it’s working on entirely in-house.
One is the monitoring of brain activity while people undergo epilepsy or brain tumor surgery. The hope is that this will help ensure surgeons don’t accidentally damage other parts of the brain.
That application should be easier to get approved by the FDA than one that would require doctors to implant the electrodes permanently, according to Vachicouras.
The second indication is tinnitus, a condition where people hear noises, such as ringing or roaring in their ears, despite no actual sounds happening. An estimated 14% of the population lives with tinnitus and, for some, it can be intolerable, making it hard to sleep, hear, or think.
Past research suggests the condition may be treatable with brain stimulation.
“Around 100 patients were tested 10 years ago … We contacted [those study leaders] to understand what went wrong, what went right, and why they stopped, and one of the big points was that the electrodes that they were using were not suitable to access these regions,” said Vachicouras.
“Having these high-resolution, soft electrodes seemed like a great way to really improve the therapy,” he added.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].






