New study shows how electricity can turn on genes
A prototype wearable has demonstrated a novel way to trigger gene expression: by zapping cells with electricity. With further development, the researchers believe it could lead to implants that help treat a variety of health issues linked to genes with a push of a button.
Gene expression 101: Most genes are like instruction manuals that tell cells how to make proteins. Nearly every cell in your body contains a copy of all your genes, but only some of them in each cell are turned on (or “expressed”) — this is what makes blood cells different from bone cells.
One way your body dictates which genes are expressed is by tagging them with chemical compounds. But because genes being on or off when they shouldn’t be can cause disease, many researchers are exploring ways to manually control gene expression in cells.
“We’ve wanted to directly control gene expression using electricity for a long time; now we’ve finally succeeded.”
Martin Fussenegger
They’ve found many ways to do this — some researchers use drugs, light, or gene-editing tech — and in 2020, a team from ETH Zurich even used electricity, demonstrating how they could engineer pancreatic cells, implanted in diabetic mice, to produce insulin when stimulated with an electric current.
“We’ve wanted to directly control gene expression using electricity for a long time; now we’ve finally succeeded,” said lead researcher Martin Fussenegger at the time.
What’s new? The team’s 2020 device consisted of a circuit board connected to a tiny capsule of engineered cells. The whole thing had to be implanted into the body, and while it worked, high-voltage signals had to be sent to the cells for hours to trigger gene expression.
The team has now developed a safer, more efficient way to trigger gene expression with electricity. They detail the approach, which they call “direct current (DC)-actuated regulation technology” (DART), in a paper published in Nature Metabolism.
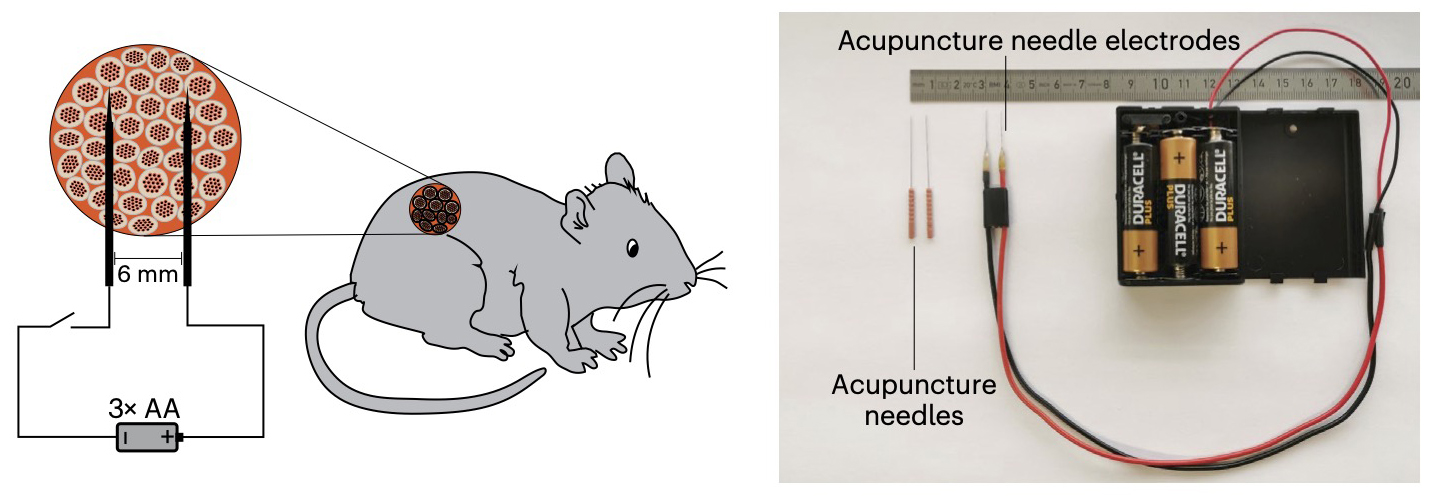
How it works: The only part of this new device that’s implanted into the body is a gel capsule containing human pancreatic cells, which were engineered so that electrical stimulation would trigger the expression of the insulin gene in them.
A pair of acupuncture needles placed in the skin then deliver low-voltage electrical stimulation from a battery pack worn outside the body. In five mouse models of diabetes, just 10 seconds of this stimulation per day triggered the cells to produce enough insulin to stabilize blood sugar levels.
Looking ahead: This study is just a proof-of-concept, and it’s not clear whether the ETH team believes it could translate to humans with diabetes — presumably, humans would need far more engineered cells to produce enough insulin to meet our daily needs, and in the 2020 study, they noted that the capsules would also need to be refilled periodically.
What is clear is that the researchers believe DART could lead to the development of wearables to control gene expression for a variety of medical issues.
“[W]e believe rapid, electronics-free direct battery-powered low-voltage DC control of therapeutic transgenes in human cells is a leap forward, representing the missing link that will enable wearables to control genes in the not-so-distant future,” they wrote in their paper.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].