New treatment “starves” aggressive brain tumors in mice
Researchers at Tel Aviv University have discovered a way to “starve” aggressive glioblastoma tumors in the brains of mice — suggesting a new way to finally fight the deadly cancer in humans.
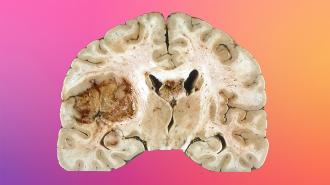
The challenge: Glioblastoma is a rare but aggressive type of brain cancer that’s almost always fatal in adults — the average survival time is just 12-18 months, and only 5% of patients live for more than five years after diagnosis.
Glioblastoma doesn’t respond to known cancer treatments, and the blood-brain barrier makes developing new ones a challenge. While the barrier is essential to prevent microorganisms in the bloodstream from reaching the brain, it also keeps meds from entering brain tumors.
Only 5% of people with glioblastoma live for more than five years after diagnosis.
The idea: When the Tel Aviv team looked at glioblastoma tumors under the microscope, they saw that they were surrounded by activated “astrocytes,” a star-shaped type of brain cell that is not itself cancerous but is linked to the progression of several brain diseases.
That inspired them to take a close look at the role astrocytes play in the growth of glioblastoma tumors in the hope it would lead to a new treatment angle.
Eyes on astrocytes: The team used a unique method to eradicate all of the active astrocytes around glioblastoma tumors in mice — and it caused their cancer to disappear within days.
All of the treated animals survived the duration of the study, while all of the untreated animals died within 4-5 weeks. The brain tumors remained gone as long as the treatment was continued, but even after it was stopped, 85% of the treated animals remained cancer-free.
“The astrocytes ‘persuade’ the immune cells to ‘change sides’ and support the tumor instead of attacking it.”
Lior Mayo
Next the team compared the gene expression of astrocytes in healthy brains to those near glioblastoma tumors and found two main differences.
The first was that the astrocytes near tumors used their ability to summon immune cells to the brain to help the cancer, rather than fight it.
“[O]nce the summoned immune cells reach the tumor, the astrocytes ‘persuade’ them to ‘change sides’ and support the tumor instead of attacking it,” said corresponding author Lior Mayo.
The second difference was that astrocytes near tumors increased their production of cholesterol — normally used to supply energy to other brain cells — and delivered it straight into the glioblastoma tumors.
“With access to energy sources in the blood barred by the blood-brain barrier, [the glioblastoma cells] must obtain this energy from the cholesterol produced in the brain itself,” said Mayo.
The team then engineered astrocytes to stop producing a protein essential for the transport of cholesterol. When those astrocytes were tested in animal models and samples taken from human patients, glioblastoma tumors were “starved” of energy and died within a few days.
Leap to humans: So far, all of the team’s research has been conducted in animals or in lab samples, so we don’t know if their results would translate to patients — but their analysis of hundreds of glioblastoma patients gives reason for hope.
“For each patient, we examined the expression levels of genes that either neutralize the immune response or provide the tumor with a cholesterol-based energy supply,” the researchers wrote.
“We found that patients with low expression of these identified genes lived longer, thus supporting the concept that the genes and processes identified are important to the survival of glioblastoma patients,” they continued.
“In the specific case of glioblastoma, the blood-brain barrier may be beneficial to future treatments.”
Lior Mayo
Looking ahead: More research is needed before the team could try to translate its findings into a therapy for people, but their study is an encouraging sign that a new kind of cancer treatment could be just on the horizon.
“Our findings suggest that, at least in the specific case of glioblastoma, the blood-brain barrier may be beneficial to future treatments, as it generates a unique vulnerability — the tumor’s dependence on brain-produced cholesterol,” said Mayo.
“We think this weakness can translate into a unique therapeutic opportunity,” he continued.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].