Experimental implant could end the need for insulin injections
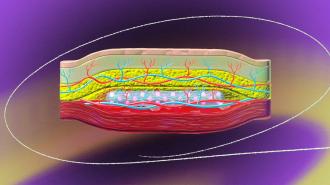
A new kind of implant could one day make it far easier for people with type 1 diabetes to manage their disease. The insulin-making implant is a mixture of transplanted islets cells and medical technology, inserted just below the skin in a person’s arm, and if it perform well in clinical trials, it could potentially last for years.
The challenge: Insulin is a hormone that our bodies use to convert sugar in our blood into energy. People with type 1 diabetes don’t produce enough (or any) insulin — if left untreated, this causes dangerously high blood sugar levels, leading to serious health issues or even death.
Regularly checking blood sugar levels and injecting synthetic insulin when they’re high is the most common way to treat type 1 diabetes, but it isn’t the only way.
People with hard-to-treat diabetes may qualify for a rare, experimental procedure in which doctors transplant insulin-producing cells, called “islets,” into their livers. The islet cells can then produce the insulin they need with fewer or no shots.
“If we could do a transplant with less or no anti-rejection drugs, we could include more patients who could benefit.”
James Shapiro
While some people have enjoyed 20+ years of insulin independence after an islet transplant, nearly 70% will be dependent on synthetic insulin again within 5 years. However, their diabetes is often easier to manage than prior to the procedure.
The tradeoff for this temporary freedom from synthetic insulin is the need to take drugs that suppress their immune system. That prevents it from attacking the donated islet cells, but it also leaves transplant recipients at high risk of infection.
“If we could do a transplant with less or no anti-rejection drugs, we could do it much more safely, and we could include more patients who could benefit,” said James Shapiro, a professor of medicine and surgical oncology at the University of Alberta (U of A).
The ideas: In 2021, a lab headed by Minglin Ma, a professor of biological and environmental engineering at Cornell University, unveiled a thin mesh tube that could be filled with islet cells and implanted in a person’s abdomen to manage their type 1 diabetes.
In tests on mice, the mesh protected the islet cells from the immune system while still allowing them to get the oxygen and nutrients they need and release insulin in response to rising blood-sugar levels. The implant worked for up to six months — no immunosuppression necessary.
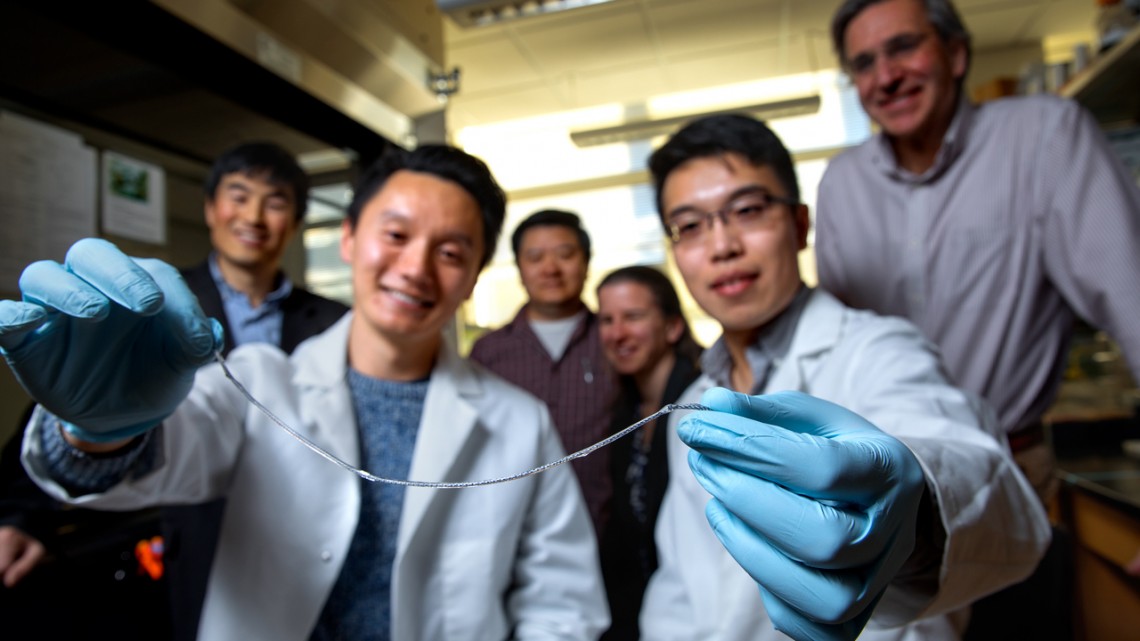
At the U of A, meanwhile, Shapiro’s team had developed a method for creating a pocket for donated islets in a person’s arm, instead of the abdomen, by inserting a plastic tube under their skin. Blood vessels then grow around the tube, and when removed, the islet cells could be inserted in the cavity.
This wouldn’t eliminate the need for immunosuppression after an islet transplant, but it would make the procedure far less invasive, and it seemed to work well in mice.
The collaboration: When Shapiro learned of the Cornell team’s device, he saw an opportunity to combine the two strategies — his method could be used to create a cavity in the arm, and Cornell’s islet-containing tube could then fill it, providing the insulin a person needed without immunosuppression.
“That channel is a perfect fit for our device. Shapiro used the analogy that this is like a hand in a glove,” said Ma. “And putting something under your skin is much easier, much less invasive than in the abdomen. It can be done as an outpatient procedure, so you don’t have to stay in the hospital. It can be done under local anesthesia.”
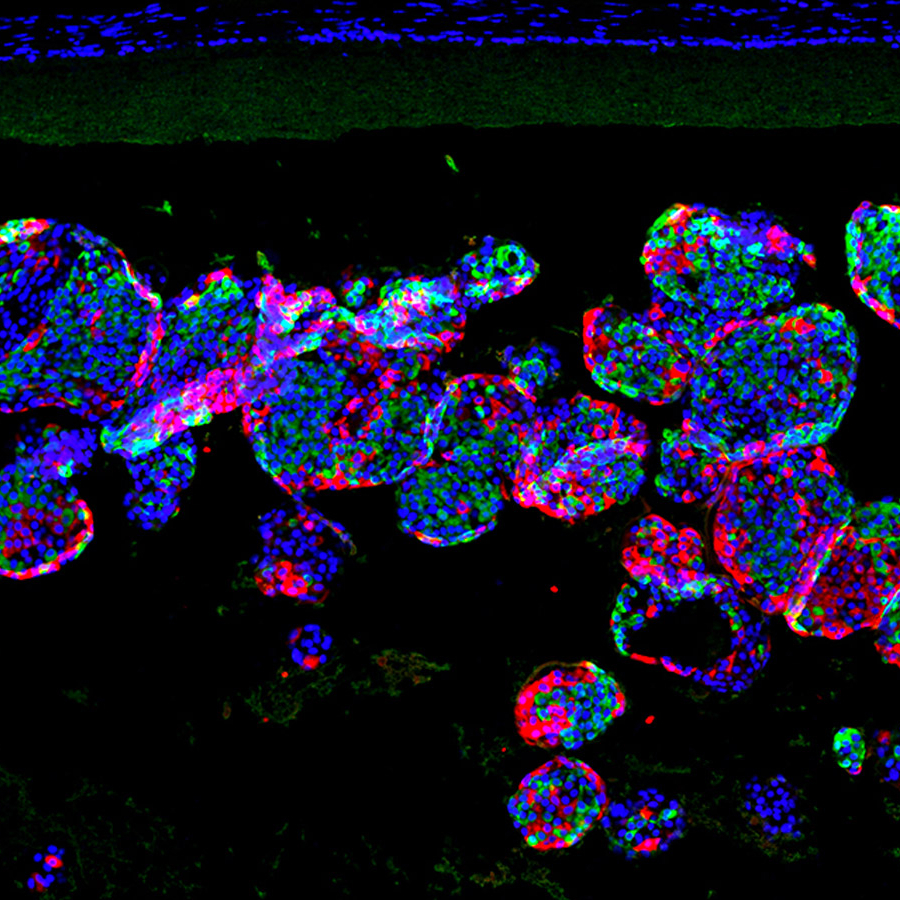
Looking ahead: The researchers have tested their device in mouse models of diabetes, where it was able to maintain some of the rodents’ insulin levels for more than six months. They also developed techniques for implanting, removing, and replacing the device using minipigs.
The team plans to continue developing their system, which they call SHEATH (Subcutaneous Host-Enabled Alginate THread), with the ultimate goal of getting the islet cells to function in a person with type 1 diabetes for 2 to 5 years before the device needs to be replaced.
“The challenge is, it’s very difficult to keep these islets functional for a long time inside of the body where you have a device, because the device blocks the blood vessels, but the native islet cells in the body are known to be in direct contact with vessels that provide nutrients and oxygen,” said Ma.
“The device is designed in a way that we can maximize the mass exchange of nutrients and oxygen,” he continued, “but we may need to provide additional means to support the cells for a long-term function in large-animal models and eventually patients.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].